【Product name】
SARS-CoV-2 Antigen Rapid Test Kit(Fluorescence immunoassay)
【Package specification】
25 Tests/kit
【Intended use】
SARS-CoV-2 Antigen Rapid Test Kit is a lateral flow immunoassay
intended for the quantitative detection of the Nucleocapsid antigen
from SARS-CoV-2 in nasopharyngeal swabs from individuals who are
suspected of SARS-COV-2 by their healthcare provider within the
first 7 days of symptom onset, or for screening of individuals
without symptoms, or other reasons to suspect SARS-COV-2 infection,
if applicable.
Positive results indicate the presence of viral antigens, but
clinical correlation with patient history and other diagnostic
information is necessary to determine infection status. Positive
results do not rule out bacterial infection or co-infection with
other viruses. The agent detected may not be the definite cause of
disease.
Negative results should be treated as presumptive, and do not rule
out SARS-CoV-2 infection and should not be used as the sole basis
for treatment or patient management decisions, including infection
control decisions. Negative results should be considered in the
context of a patient’s recent exposures, history, and the presence
of clinical signs and symptoms consistent with SARS-CoV-2, and
confirmed with a molecular assay, if necessary, for patient
management.
The SARS-COV-2 Antigen Rapid Test Kit is intended for use by
trained clinical laboratory personnel specifically instructed and
trained in vitro diagnostic procedures.
【Inspection principle】
The SARS-CoV-2 Nucleocapsid proteins Rapid Test is a one-step
chromatographic sandwich immunoassay designed for the quantitative
measurement of SARS-CoV-2 Nucleocapsid proteins. The SARS-CoV-2
Nucleocapsid proteins in the sample was first bound with the
conjugated compound of fluorescent labeled SARS-CoV-2 Nucleocapsid
proteins monoclonal antibody, then moved and combined with another
SARS-CoV-2 Nucleocapsid proteins monoclonal antibody fixed on the
nitrocellulose membrane, and the double antibody sandwich complex
was formed at the detection line of the cellulose nitrate membrane.
The quantitative detection results were obtained by NIR-1000 dry
fluoroimmunoassay analyser.
【Components】
| Name | Quantity | Component |
| Test cards | 25 | It is composed of fluorescent pad (coated with fluorescent labeled
SARS-CoV-2 Nucleocapsid proteins monoclonal mouse antibody),
nitrocellulose membrane (coated with SARS-CoV-2 Nucleocapsid
proteins monoclonal mouse antibody and Goat anti mouse IgG
antibody), absorbent paper and backing |
| Sample diluent | 25(400μL/tube) | Phosphate buffer |
| Nasal Mid-Turbinate (NMT) swabs | 25 | Flocking |
| ID card | 1 | With specific stand curve file |
The components in different batches of kits cannot be used
interchangeably.
【Storage conditions and validity】
The kit should be stored at 4℃~30℃, out of direct sunlight. It is
valid for 18 months. The test card should be used within 15 minutes
after unsealing under the environment of 15℃~30℃ and 20% ~ 90%
relative humidity.
The production date, batch number and expiration date are shown in
the outer package of the product.
【Applicable instruments】
Nir-1000 dry fluorescent immunoassay analyzer produced by WWHS
Biotech. Inc.
【Sample requirements】
1.Nasal Mid-Turbinate (NMT) swabs can be used for testing.Venous
blood was collected according to routine laboratory methods to
avoid hemolysis.
2.Nasal Mid-Turbinate (NMT) swab specimens need to be tested
immediately and within 30 minutes of collection.
3.Specimens can be stored at room temperature (15-30 °C) for up to
60 minutes until testing.
【Interpretation of results】
1.This reagent is only used for auxiliary detection. If the test
results are abnormal, it should be reviewed in time and judged in
combination with clinical symptoms.
2.For samples with SARS-CoV-2 Nucleocapsid proteins concentration
lower than 0.025ng/ml and higher than 500ng/ml, the detection
results are reported as "< 0.025ng/ml" and "> 500ng /ml",
respectively.
【Note】
- For in vitro diagnostic use.
- This test has been authorized only for the detection of SARS-CoV-2
antigen, not for any other viruses or pathogens.
- Treat all specimens as potentially infectious. Follow universal
precautions when handling samples, this kit and its contents.
- Proper sample collection, storage and transport are essential for
correct results.
- Leave test card sealed in its foil pouch until just before use. Do
not use if pouch is damaged or open.
- Do not use kit past its expiration date.
- Do not mix components from different kit lots.
- Do not reuse the used test card.
- Inadequate or inappropriate sample collection, storage, and
transport may yield false test results.
- Do not store specimens in viral transport media for specimen
storage.
- All components of this kit should be discarded as Biohazard waste
according to Federal, State and local regulatory requirements.
- Solutions used to make the positive control swab are
non-infectious. However, patient samples, controls, and test cards
should be handled as though they could transmit disease. Observe
established precautions against microbial hazards during use and
disposal.
- Wear appropriate personal protection equipment and gloves when
running each test and handling patient specimens. Change gloves
between handling of specimens suspected of SARS-COV-2.
- INVALID RESULTS can occur when an insufficient volume of extraction
reagent is added to the test card. To ensure delivery of adequate
volume, hold vial vertically, ½ inch above the swab well, and add
drops slowly.
- False Negative results can occur if the sample swab is not rotated
(twirled) prior to closing the card.
- Swabs in the kit are approved for use with SARS-COV-2 Ag Card. Do
not use other swabs.
- The Extraction Buffer packaged in this kit contains salts,
detergents and preservatives that will inactivate cells and virus
particles. Samples eluted in this solution are not suitable for
culture.
- Do not store the swab after specimen collection in the original
paper packaging, if storage is needed use a plastic tube with cap.


Covid-19 New Self-test Rapid Ag Test
Kit

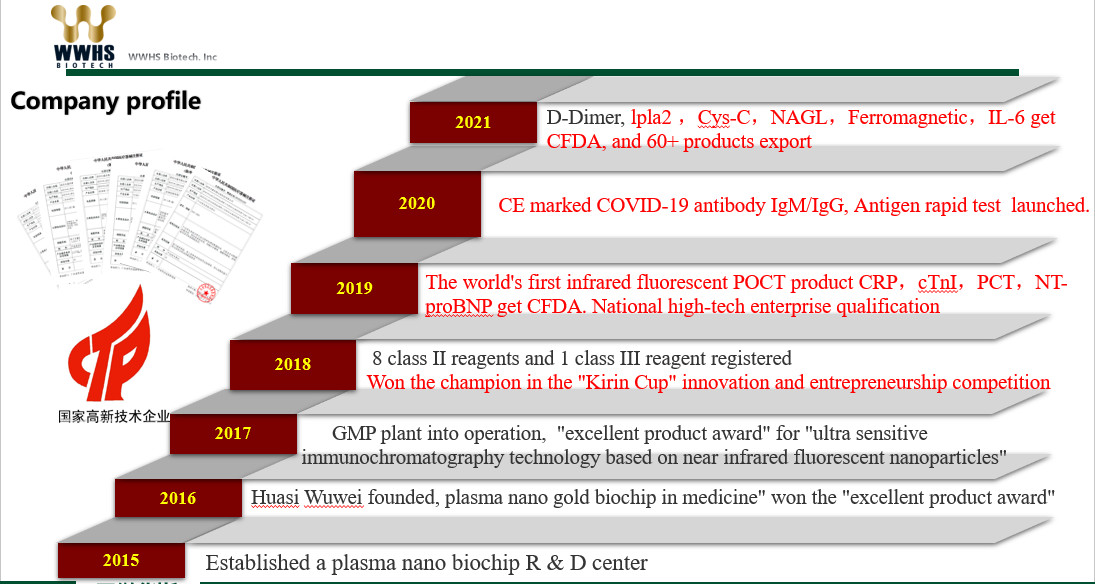
About WWHS Biotech Inc
WWHS Biotech. Inc Is A High-Tech Enterprise Incubated By The
Research Institute Of Tsinghua University In Shenzhen In 2016. The
Company Is Located In The National Biomedical Industrial Base In
Pingshan District, Shenzhen, With About 32,000 Square Feet Space
For Workshop And Office.
As A Fast-Growing Biotechnology Company, We Dedicate To The
Development Of World-Class Bio-Detection Technologies That Meet The
Ever-Increasing Demands On Clinical Diagnostics. We Focus On
Developing Cost-Effective And Precise Rapid Diagnostic Products
Based On Our Unique Near Infrared (NIR) Fluorescence Platform
Technologies (PGOLD™ And IR-LF™) With Major Interests In
Cardiology, Infectious Diseases, Oncology, Metabolic Diseases And
Women And Children’s Health.
Limitations of methods
1. This kit is only used to detect human plasma/whole blood samples
2. Due to the limitations of immunoassay methods of antigen and
antibody reaction, the results cannot be used as the only basis for
clinical diagnosis, but should be evaluated with all the existing
clinical and experimental data.
3. The content of triglyceride in the sample shall not exceed
15mg/ml, the content of hemoglobin shall not exceed 5mg/ml, and the
content of bilirubin shall not exceed 0.5mg/ml, and the relative
deviation of the test results shall not exceed ±15%.
4. When the concentration of AFP in the sample is less than
20000ng/ml, there is no hook effect.
5. HAMA effect was not produced when the concentration of human
anti rat in the sample was less than 50ng/ml.
6. When RF concentration in the sample is less than 2000IU/ml, the
relative deviation of the test results is within ±15%.














